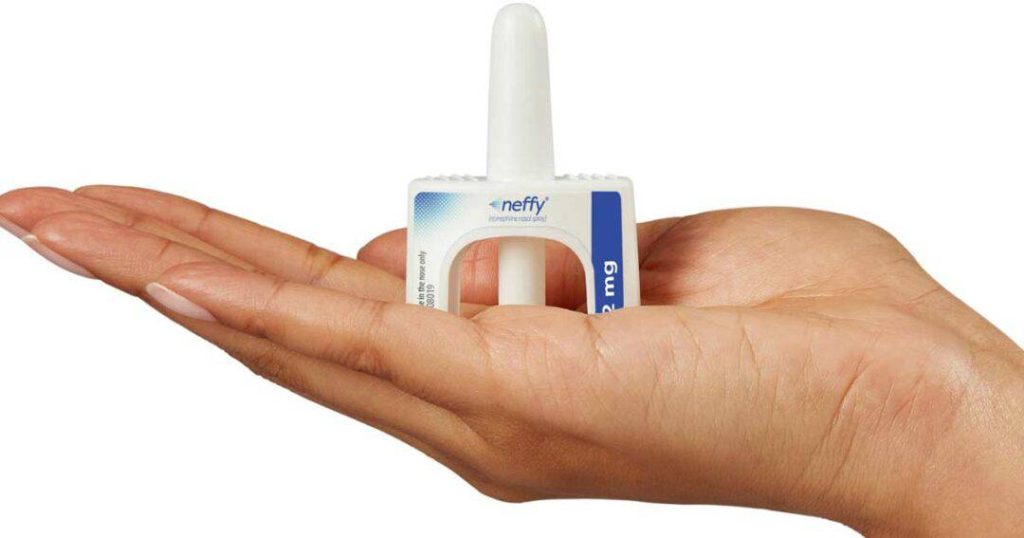
Neffy: A Needle-Free Alternative to Auto-Injectors
In a ground breaking development, Neffy has emerged as the first needle-free system alternative to traditional auto-injectors like the EpiPen. This innovative nasal epinephrine delivery system has filed for approval with Health Canada in November 2024, with advocates hopeful for approval in 2025. Upon approval, Neffy will be distributed by ARS Pharma in Canada, offering a new, convenient option for those in need of emergency epinephrine.
Neffy is not only making strides in Canada but also in the United Kingdom. EURONeffy is currently awaiting approval from UK health authorities. Both Canada and the United Kingdom will have access to 2 mg and 1 mg nasal epinephrine sprays, designed for use in children aged four years and older. To be eligible for this treatment, patients must weigh a minimum of 33 pounds (15 kg).
The introduction of Neffy represents a significant advancement in the field of emergency medicine. Traditional auto-injectors, while effective, can be intimidating and difficult to use, especially for children. Neffy’s needle-free nasal spray offers a less invasive and more user-friendly alternative, potentially increasing compliance and reducing anxiety associated with epinephrine administration.
Neffy has already received approval from the U.S. Food and Drug Administration (FDA), setting a precedent for its efficacy and safety. This approval marks a crucial step in making needle-free epinephrine delivery widely available, providing a promising option for individuals with severe allergies.
As Neffy awaits approval in Canada and the United Kingdom, the anticipation builds for a future where emergency epinephrine administration is simpler and more accessible. The potential impact of Neffy on the lives of those with severe allergies is profound, offering hope for a safer and more comfortable method of treatment.